Report Code: 12187 | Available Format: PDF | Pages: 108
Asia-Pacific Gene Therapy Market Research Report: Type (In Vivo, Ex Vivo), Vector Type (Adenovirus, Non-Viral, Herpes, Simplex Virus), Application (Carcinoma, Nasopharyngeal, Cancer, Acute Lymphoblastic Leukemia, Critical Limb Ischemia, Melanoma), End User (Pharmaceutical and Biotechnology Companies, Academic Institutes and Research Centers) - Industry Analysis and Demand Forecast to 2030
- Report Code: 12187
- Available Format: PDF
- Pages: 108
- Report Description
- Table of Contents
- Market Segmentation
- Request Free Sample
Market Overview
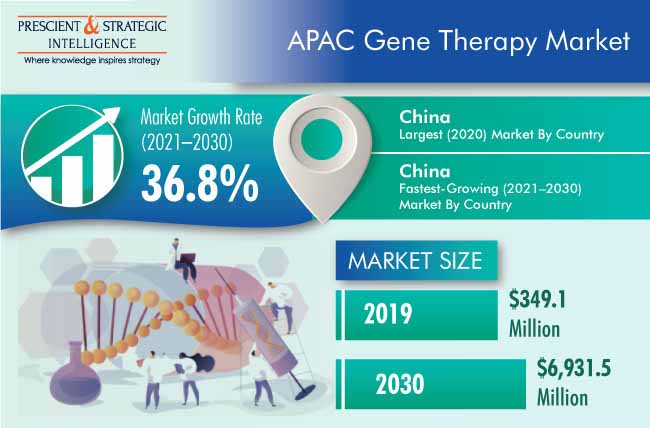
The Asia-Pacific (APAC) gene therapy market valued $349.1 million in 2020, which is projected to progress at a CAGR of 36.8% during the forecast period (2021–2030). The major factors responsible for the growth of the market include the increasing gene therapy development activities, surging burden of chronic diseases, and growing number of clinical trials with positive results.
Due to the current COVID pandemic, governments worldwide have implemented strong measures, such as lockdowns and social distancing, to contain the emanating health threats. Manufacturers across APAC, including those of gene therapies, have been moderately impacted by the economic crisis triggered by the pandemic. Clinical trials and the related activities were halted for a limited time in 2020 in China and Japan, thus having a moderately negative impact. However, by the end of 2020, the development of such therapies resumed, thus leading to an increase in their adoption.
Due to Less-Cumbersome Administration Process, In-Vivo Category To Dominate Market
The in-vivo category held the larger share in the gene therapy market during the historical period (2015–2020), and it is expected to retain its position in the upcoming years, based on type. This is attributed to the less-cumbersome administration process of in-vivo therapies compared to ex-vivo gene therapy, which requires the removal of cells out of the patient’s body, to be genetically modified.
Due to High Levels of Expression, Adenovirus Category To Witness Fastest Growth
The adenovirus category is expected to witness the fastest growth during the forecast period, based on vector type. This will mainly be due to the high level of expressions and high transduction efficiency of such vectors.
Owing to Increasing Number of Cancer Patients, Carcinoma Category Led Market
The carcinoma category dominated the gene therapy market in the region, based on application. According to the Global Cancer Observatory (GLOBOCAN), 2.3 million cases of cancer were reported in Southeast Asia and 1.4 million people lost their lives due to it in 2020. Also, the wide range of portfolio of drugs available to treat cancer has created more demand of such therapies such as Gendicine, which was the first approved gene therapy, in the region.
Due to Rising Research and Development Investment, Pharmaceutical and Biotechnology Companies Dominated Market
The pharmaceutical and biotechnology companies’ category held the larger share under the end user segment of the gene therapy industry during the historical period, and it is expected to maintain the lead in the coming years. This will majorly be due to the rising R&D investments, along with the active use of gene therapy for a number of disease applications.
Owing to Increasing Number of Strategic Developments among Key Companies, China Led Market
China generated the highest revenue in the gene therapy industry in 2020, and it is expected to maintain its position in the coming years. This is majorly ascribed to the rising number of strategic developments among key companies and increasing incidence of cancer and rare diseases. For instance, in March 2019, Merck & Co. Inc. signed a non-binding memorandum of understanding (MoU) with GenScript Biotech Corporation, a Chinese biotech company, for a strategic alliance focusing on plasmid and viral vector manufacturing. The alliance is helping accelerate the commercialization of cell and gene therapies in China.
Increasing Number of Product Launches and Grants
The gene therapy market is witnessing rapid evolution with the development and launch of new products. For instance, in January 2021, Amgen Inc. received the Breakthrough Therapy Designation (BTD) for its investigational KRASG12C inhibitor, Sotorasib, from the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China. The designation allows for the treatment of patients with KRAS G12C-mutated locally advanced or metastatic non-small-cell lung cancer (NSCLC), who have received at least one prior systemic therapy.
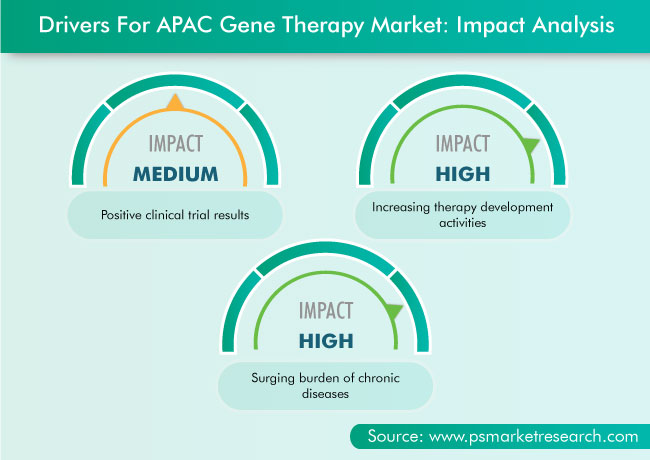
Increasing Number of Positive Clinical Trial Results Is Boosting Market Growth
The recent positive results of gene therapy studies and clinical trials are likely to encourage biopharmaceutical companies to focus on entering the gene therapy market. For instance, in May 2020, Applied Genetic Technologies Corporation (AGTC) announced the positive interim Phase I/II clinical trial results, which helped decide the dose to be used in its ongoing clinical trial on patients with X-linked retinitis pigmentosa (XLRP) due to mutations in the RPGR gene. The data demonstrated that the company’s proprietary adeno-associated viruses (AAV) vector and engineered RPGR constructs were well tolerated and had beneficial effects on the markers of the disease in a canine model of XLRP. Moreover, the patients treated showed significant improvement in visual function.
Increasing Therapy Development Activities Are Driving Market
The increasing participation of public and private research organizations in gene therapy research is boosting the growth of the gene therapy market in APAC. Due to the rapid technological advancements over decades, the development of gene therapies has regained significant interest. This process is being accomplished by the optimization of vectors, such as retroviruses and lentiviruses, by the introduction of new techniques, such as induced pluripotent stem cells, in combination with the current models of gene editing and even by trials in germ cells. Therefore, with the increasing number of research activities, the demand for gene therapy has increased.
| Report Attribute | Details |
Historical Years |
2015-2020 |
Forecast Years |
2021-2030 |
Base Year (2020) Market Size |
$349.1 Million |
Market Size Forecast in 2030 |
$6,931.5 Million |
Forecast Period CAGR |
36.8% |
Report Coverage |
Market Trends; Revenue Estimation and Forecast; Segmentation Analysis; Regional Breakdown; Competitive Analysis; Companies’ Strategic Developments; Company Profiling |
Market Size by Segments |
By Type; By Vector Type; By Application; By End User; By Country |
Market Size of Geographies |
China; Japan; Australia |
Secondary Sources and References (Partial List) |
All India Biotech Association; American Society of Gene and Cell Therapy; BioPartner UK; Center for Drug Evaluation and Research; European Biotechnology Network; Japan Society of Gene Therapy; Netherlands Society of Gene and Cell Therapy; Biotechnology Innovation Organization |
Explore more about this report - Request free sample
Market Players Are Focusing on Product Launches to Gain Competitive Edge
The APAC gene therapy market has the presence of various key players, such as Novartis AG, Sibiono GeneTech Co. Ltd., Shanghai Sunway Biotech Co. Ltd., Kolon Life Science Inc., AnGes Inc., and Amgen Inc.
In recent years, players in gene therapy industry have undertaken product launches to offer a better portfolio than other players.
- In January 2021, Amgen Inc. received the Breakthrough Therapy Designation (BTD) for its investigational KRASG12C inhibitor, Sotorasib, from the CDE, under the NMPA of China.
- InSeptember 2019, AnGes Inc. announced the launch of Collategene for the treatment of critical limb ischemia in Japan. In March 2019, this gene therapy had received the conditional approval (Approval with Conditions and Time Limit) from the Japanese Ministry of Health, Labour and Welfare (MHLW).
Key Players in Gene Therapy Industry Include:
-
Novartis AG
-
Sibiono GeneTech Co. Ltd.
-
Shanghai Sunway Biotech Co. Ltd.
-
Kolon Life Science Inc.
-
AnGes Inc.
-
Amgen Inc.
Market Size Breakdown by Segment
The Asia-Pacific gene therapy market report offers comprehensive market segmentation analysis along with market estimation for the period 2015–2030.
Based on Type
- In Vivo
- Ex Vivo
Based on Vector Type
- Adenovirus
- Non-Viral
- Herpes Simplex Virus (HSV)
Based on Application
- Carcinoma
- Nasopharyngeal Cancer
- Acute Lymphoblastic Leukemia (ALL)
- Critical Limb Ischemia
- Melanoma
Based on End User
- Pharmaceutical and Biotechnology Companies
- Academic Institutes and Research Centers
Geographical Analysis
- Japan
- China
- Australia
In 2030, $6,931.5 million will be generated by the APAC market for gene therapy.
In the APAC gene therapy industry, in-vivo therapies are preferred.
The best opportunities for investments in the APAC market for gene therapy are available in China.
The APAC gene therapy industry is growing because of the rising incidence of chronic diseases and increasing number of R&D activities and clinical trials.
Product launch is the strongest strategy among players in the APAC market for gene therapy.
Want a report tailored exactly to your business strategy?
Request CustomizationWant an insight-rich discussion with the report author?
Speak to AnalystOur dedication to providing the most-accurate market information has earned us verification by Dun & Bradstreet (D&B). We strive for quality checking of the highest level to enable data-driven decision making for you
Our insights into the minutest levels of the markets, including the latest trends and competitive landscape, give you all the answers you need to take your business to new heights
With 24/7 research support, we ensure that the wheels of your business never stop turning. Don’t let time stand in your way. Get all your queries answered with a simple phone call or email, as and when required
We take a cautious approach to protecting your personal and confidential information. Trust is the strongest bond that connects us and our clients, and trust we build by complying with all international and domestic data protection and privacy laws