Report Code: 12786 | Available Format: PDF | Pages: 280
Stem Cell Therapy Market Size and Share Analysis by Type (Allogeneic, Autologous), Cell Source (Adipose Tissue, Bone Marrow, Umbilical Cord), Application (Musculoskeletal Disorders, Wound & Injuries, Cardiovascular Diseases, Inflammatory & Autoimmune Diseases, Neurological Disorders) - Global Industry Demand Forecast to 2030
- Report Code: 12786
- Available Format: PDF
- Pages: 280
- Report Description
- Table of Contents
- Market Segmentation
- Request Free Sample
Stem Cell Therapy Market Overview
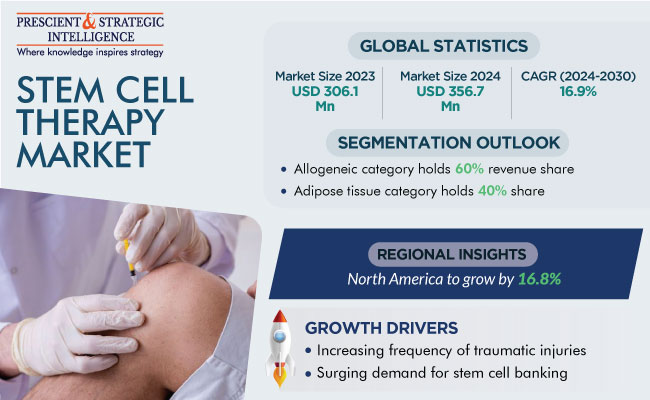
The global stem cell therapy market size was USD 306.1 million in 2023, and it is expected to reach USD 910.8 million in 2030, with a growth rate of 16.9% during 2024–2030. The major factor contributing to the market advance is the increasing frequency of traumatic injuries, orthopedic conditions, neurological diseases, and autoimmune diseases, including multiple sclerosis, lupus, COPD, Parkinson’s disease, stroke, and amyotrophic lateral sclerosis (ALS).
Moreover, with the rising knowledge of stem cell therapy, the surging demand for stem cell banking is expected to drive the market during the forecast period. Additionally, the increasing breakthroughs in research and a growing focus on customized medications are set to propel the market in the coming years.
For instance, in November 2022, the California Institute for Regenerative Medicine invested USD 8 million in the UC San Diego Alpha Stem Cell Clinic to allow the latter to create more-effective stem cell therapies for difficult-to-treat diseases.
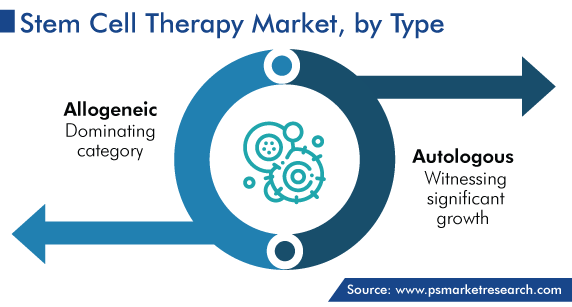
Allogeneic Stem Cell Therapy Accounted for Largest Share of Market
Allogeneic stem cell therapy held the largest share, of 60%, in 2023, and it is expected to grow at a CAGR of 17.0% during 2024–2030. This is attributed to the increase in the number of clinical trials for the development of such treatments. The biggest advantage of allogeneic transplantation is that the graft does not have any contaminating tumor cell. The graft also contains immunocompetent cells derived from a donor, which may initiate an immune graft-versus-malignancy effect.
Moreover, many studies on stem cell transplants have shown promising results. For instance, a study published in Clinical Cancer Research, a journal of the American Association for Cancer Research, in January 2023 states that an investigational allogeneic T-cell therapy, which simultaneously targets six different viruses, has shown promising safety and efficacy in the phase II clinical trial on patients who had undergone stem cell transplantation to treat cancer and certain blood-related diseases.
Similarly, leukemia patients treated with allogeneic stem cells often get acute graft-versus-host disease. In this condition, the immune cells of the transplanted tissue become hyperactive and damage the recipient’s healthy tissue.
Moreover, an analysis conducted at the Norris Comprehensive Cancer Center and presented at the 64th annual meeting of the American Society of Hematology mentioned significant positive outcomes in patients with acute myeloid leukemia who were treated with FMS-like tyrosine 3 (FLT3) inhibitors after a hematopoietic stem cell transplant.
Furthermore, in January 2023, Jasper Therapeutics Inc., a biotechnology company, announced that the first three participants in a phase I/II clinical trial to evaluate the augmentation of an existing regiment for bone marrow transplantation with briquilimab have shown positive clinical results. This approach is designed to enable the grafting of a higher percentage of healthy stem cells from a donor, without increased toxicity. The study is led by the Cellular and Molecular Therapeutics Laboratory at the NHLBI. The first two participants with peripheral blood chimerism after allogeneic stem cell transplant achieved 100% donor myeloid chimerism.
Recent studies also support the efficacy of allogeneic stem cell transplants. For instance, in September 2022, Duke Cancer Institute discussed why a 10-year follow-up of a study of patients who had received an allogeneic stem cell transplant (ASCT) with omidubicel is significant. Many patients face challenges in finding an unrelated donor for an ASCT. In phase III trials, Omidubicel, which is an umbilical-cord-blood-derived hematopoietic stem cell graft, has shown to be more effective than the conventional myeloablative umbilical cord blood. Moreover, the FDA has undertaken a priority review for the approval of this therapy for hematologic malignancy patients who require an ASCT.
Moreover, in November 2022, Gamida Cell Ltd. provided an update on recent interactions with the FDA related to the Biologics License Application (BLA) for omidubicel, the company’s advanced cell therapy candidate for allogeneic hematopoietic stem cell transplantation.
The autologous therapy category is projected to witness significant growth during the forecast period because of the low risk of graft rejection in this treatment. People who need high-dose of radiation and chemotherapy are often transplanted with autologous stem cells to replace the bone marrow damaged by the primary treatment.
Moreover, numerous recent studies focusing on autologous hematopoietic stem cell transplants (HSCT) have shown positive results for secondary progressive multiple sclerosis. The approach uses healthy blood stem cells from a person’s own body to replace diseased cells.
Adipose Tissue Category Accounted for Largest Share
The adipose tissue category dominated the market, with a share of 40%, in 2023. It is because of the numerous advantages of adipose-tissue-derived stem cells, such as the convenience of harvesting them using minimally invasive techniques and the simplicity of the isolation approach.
In addition, adipose-tissue-derived stem cells (ASCs) have the potential to differentiate into several other cells, such as chondrocytes, adipocytes, and osteoblasts and the ability to act as immunomodulators, by stimulating the migration of immune cells to the damaged tissues. Moreover, they are easy to obtain and have a high proliferation rate, low donor site morbidity, and excellent differentiation abilities. Research on the optimal preparation process for ASCs and their application in various disorders continues to advance.
Moreover, companies are using these biological agents for clinical trials. For instance, in March 2022, American CryoStem Corporation, a clinical-stage biotech firm, reached the midpoint of the phase I clinical trial of its ATCell autologous mesenchymal stem cell therapy for post-concussion syndrome (PCS).
Similarly, companies are publishing the results of studies regarding adipose-derived regenerative cells. For instance, in January 2023, InGeneron Inc., a clinical-stage biotechnology company, announced the publication of a study revolving around the usage of InGeneron’s Transpose RT system for the characterization of the composition of regenerative cells extracted from 232 subjects’ adipose tissue.
Stem Cell Therapy Is Majorly Used for Musculoskeletal Disorders
The musculoskeletal disorders category, within the application segment, generated the highest revenue in 2023. This is attributed to the increasing prevalence of musculoskeletal disorders, such as carpal tunnel syndrome, muscle strains, lower-back injuries, and osteoarthritis. According to the WHO, approximately 1.71 billion people have a musculoskeletal condition globally.
InGeneron Inc. published a succinct scientific review of stem cell therapy in January 2022 in the Journal of Orthopaedic Surgery and Research. The publication gives an approachable overview of the stem cell biology and clarifies common misconceptions about adipose-derived regenerative cells. The authors emphasize the ability of therapies using ADRCs to readily fit into modern orthopedic treatment concepts and the reference InGeneron’s cell therapy platform, currently under evaluation in ongoing FDA-approved trials.
| Report Attribute | Details |
Market Size in 2023 |
USD 306.1 Million |
Market Size in 2024 |
USD 356.7 Million |
Revenue Forecast in 2030 |
USD 910.8 Million |
Growth Rate |
16.9% CAGR |
Historical Years |
2017-2023 |
Forecast Years |
2024-2030 |
Report Scope |
Market Trends, Drivers, and Restraints; Revenue Estimation and Forecast; Segmentation Analysis; Impact of COVID-19; Companies’ Strategic Developments; Market Share Analysis of Key Players; Company Profiling |
Segments Covered |
By Type; By Cell Source; By Application; By Region |
Explore more about this report - Request free sample
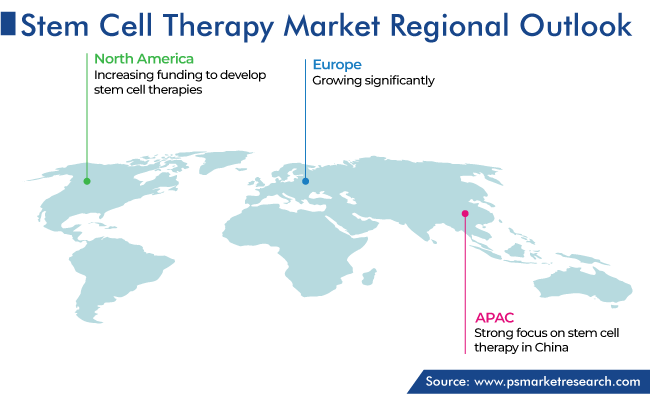
North America Held Largest Share of Stem Cell Therapy Market
North America accounted for the largest share, of 55%, in 2023, and it is expected to grow at a significant CAGR during 2024–2030. This is credited to the funding being given to develop stem cell therapies in this region. For instance, in September 2022, Forge Biologics Inc., a gene-therapy-focused contract development and manufacturing organization, announced that it has raised USD 90 million in a Series C financing round co-led by Drive Capital and Aisling Capital, with an additional undisclosed strategic investor.
Moreover, companies are focusing on combination agreements. For instance, in August 2022, Roslin Cell Therapies Limited, a contract development and manufacturing organization focused on cell and gene therapies based in Edinburgh’s BioQuarter; and Lykan Bioscience LLC, a cell-based-therapy-focused CDMO, signed a business combination agreement. The combined group will provide cGMP manufacturing services and process development expertise for an extensive variety of autologous and allogeneic cell therapies, apart from gene editing and induced pluripotent stem cells.
Asia-Pacific is the fastest-growing region in the market, with China being a major country in the region focusing on stem cell therapy. For instance, in January 2023, OBiO Technology Corp. Ltd., a Shanghai-based gene-and-cell-therapy-focused CRO and CDMO, announced that the FDA has accepted its GMP plasmid drug master file (DMF). The DMF acceptance allows direct references for investigational new drug filings for allogeneic and autologous cell therapy and stem cell therapy products and helps shorten the time involved in communication, evaluation, and review of the filings for related therapeutics on part of the FDA.
Some Key Players Operating in Market Are:
- Smith & Nephew plc
- MEDIPOST Co. Ltd.
- Anterogen Co. Ltd.
- CORESTEM Inc.
- PHARMICELL Co., Ltd
- NuVasive Inc.
- RTI Surgical Inc.
- AlloSource
- JCR Pharmaceutical Co. Ltd.
- Takeda Pharmaceutical Company Limited
Market Size Breakdown by Segment
The report analyzes the impact of the major drivers and restraints on the stem cell therapy market, to offer accurate market estimations for 2017-2030.
Based on Type
- Allogeneic
- Autologous
Based on Cell Source
- Adipose Tissue
- Bone Marrow
- Umbilical Cord
Based on Application
- Musculoskeletal Disorders
- Wound & Injuries
- Cardiovascular Diseases
- Inflammatory & Autoimmune Diseases
- Neurological Disorders
Geographical Analysis
- North America
- U.S.
- Canada
- Europe
- Germany
- U.K.
- France
- Italy
- Spain
- Asia-Pacific
- Japan
- China
- India
- South Korea
- Australia
- Latin America
- Brazil
- Mexico
- Middle East and Africa
- Saudi Arabia
- South Africa
- U.A.E.
In 2030, the market for stem cell therapies will value USD 910.8 million.
The allogeneic type dominates the stem cell therapy industry.
The market for stem cell therapies is majorly driven by the huge R&D investments.
Musculoskeletal disorders are the most significant in the stem cell therapy industry.
APAC is the most-lucrative market for stem cell therapies.
Want a report tailored exactly to your business strategy?
Request CustomizationWant an insight-rich discussion with the report author?
Speak to AnalystOur dedication to providing the most-accurate market information has earned us verification by Dun & Bradstreet (D&B). We strive for quality checking of the highest level to enable data-driven decision making for you
Our insights into the minutest levels of the markets, including the latest trends and competitive landscape, give you all the answers you need to take your business to new heights
With 24/7 research support, we ensure that the wheels of your business never stop turning. Don’t let time stand in your way. Get all your queries answered with a simple phone call or email, as and when required
We take a cautious approach to protecting your personal and confidential information. Trust is the strongest bond that connects us and our clients, and trust we build by complying with all international and domestic data protection and privacy laws