Market Statistics
| Study Period | 2019 - 2030 |
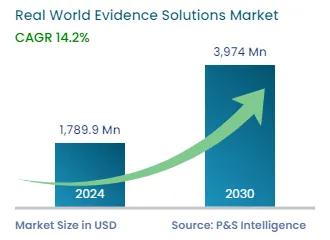
| 2024 Market Size | USD 1,789.9 Million |
| 2030 Forecast | USD 3,974 Million |
| Growth Rate(CAGR) | 14.2% |
| Largest Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Nature of the Market | Fragmented |
Report Code: 12563
Get a Comprehensive Overview of the Real World Evidence Solutions Market Report Prepared by P&S Intelligence, Segmented by Component (Services, Data Sets), Application (Drug Development and Approvals, Post-Market Surveillance, Market Access Reimbursement/Coverage Decision Making, Clinical and Regulatory Decision-Making), End User (Pharmaceutical & Medical Device Companies, Healthcare Providers, Healthcare Payers), and Geographic Regions. This Report Provides Insights from 2019 to 2030.
| Study Period | 2019 - 2030 |
| 2024 Market Size | USD 1,789.9 Million |
| 2030 Forecast | USD 3,974 Million |
| Growth Rate(CAGR) | 14.2% |
| Largest Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Nature of the Market | Fragmented |
Explore the market potential with our data-driven report
The global real world evidence solutions market size stood at USD 1,789.9 million in 2024, and it is expected to advance at a compound annual growth rate of 14.2% between 2024 and 2030, to reach USD 3,974 million by 2030. This is due to the rising number of clinical trials, the shifting focus to value-based healthcare from volume-based, and the surging R&D activities related to the development of drugs and medical devices.
Moreover, RWD and RWE are used to improve protocol design, which will lead to less the number of costly protocol revisions and can also be used to create synthetic control arms, which will speed up trial execution and reduce the overall cost. The continuous development in data variety, volume, and speed, and the need for quick delivery of insights drawn from that data are boosting the demand for enhancement of the right technologies for real-world evidence program implementation. Thus, several life sciences companies are adopting cloud technologies because of the flexibility, speed, safety, and scalability they offer.
Several biopharmaceuticals, drug, and medical device organizations are heavily investing in R&D for the creation of novel drugs and gadgets. These companies are selecting fully functional or integrated outsourcing services, from the early to the late-stage development phase, to encounter the needs for both drug discovery and development. Among all, the pharmaceutical sector spends the highest portion of its income on R&D. Thus, the rising R&D spending and the growing demand for preclinical and clinical services during the development process of drugs are driving the need for real-world evidence solutions.
The healthcare network is transforming and there is amplified analysis on healthcare costs, as healthcare shareholders are observing for innovative solutions to cater to the unaffordable cost burden and comparatively low return on investment. Also, an opportunity has been created for an end-to-end approach to leverage the proof, data, and knowledge assets of a life sciences organization, which is assisting in dismantling traditional silos and allowing insight-driven supervisory from the phase of products’ research and development to their commercialization. This is implementing a strong governance plan, with the help of technologies, like self-service and cloud analytics, and gaining the aptitude to combine data sets and comprehend the suitable resources for the essential analytics.
The services category accounted for a larger revenue share, of over 56%, in 2022, and it is also expected to maintain its dominance throughout the forecast period. This is ascribed to the reducing drug development delays, which is now more important than ever before, and the availability of a higher amount of healthcare information. Moreover, companies frequently adapt the necessary services as per the client's needs, which saves time and money compared to obtaining the data sets and analyzing them to generate helpful information.
Also, real-world evidence program professionals communicate and work together with the subject matter professionals of medical device and pharmaceutical companies to provide services for the development of intelligent RWE plans and use their analytical abilities to offer valuable statistical insights.
The data sets category is projected to grow at a significant growth rate during the forecast period. The growth can be primarily ascribed to the rising amount of information generated in the healthcare sector and the increasing attention to more insights into epidemiology. Additionally, clinical data is an important source of most health and medical research that is either collected from the current patient care or formal clinical trial programs.
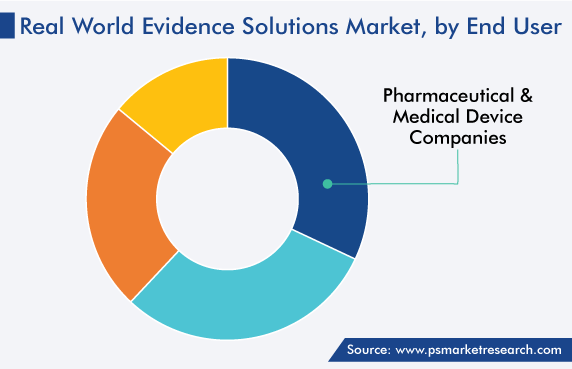
The pharmaceutical & medical device companies category is projected to grow at the highest CAGR, of 12.9%, during the forecast period. This can be primarily ascribed to the rising importance of RWE analyses in drug support, the valuation of drug performance in real-world settings, and the inhibition of costly drug recalls.
In addition, RWE is increasingly being used in the pharmaceutical sector to fulfill the governing compliance necessities as well as payer requirements related to HEOR. Pharmaceutical enterprises need information describing both the medically accepted and approved alternative uses of formerly approved drugs. This solution data is required for new medicines to effectively pass via clinical phases. Therefore, a thriving phase shift is reliant on strong real-world results.
Drive strategic growth with comprehensive market analysis
The APAC market is expected to grow at the highest CAGR, of around 14%, in the upcoming years. The growth can be mainly ascribed to the rising government support for the adoption of RWE studies, the surging number of clinical trials, the increasing prevalence of chronic diseases, the presence of many contract research organizations, the mounting demand for advanced healthcare services, and the growing geriatric population in the region. Moreover, some regional countries work to completely embrace real-world evidence. For instance, Japan has started its “Rational Medicine” to make the Japanese healthcare system more patient-centric and evidence-based.
In India, the healthcare sector is rapidly growing and understanding the laws of health economics and research outcomes, thereby demonstrating the value of real-world insight into healthcare decisions. India has taken a step toward RWE by creating a framework to help healthcare providers optimize RWD for clinical, economic, and humanistic results. For this, the Indian branch of ISPOR has already drafted the proposed PE guidelines.
China is significantly moving toward the use of RWE. The demand for such solutions is expected to rise because many centers are doing research in the country. For instance, the Shanghai Clinical Center for Endocrine and Metabolic Diseases has completed many studies on non-communicable diseases and the risk factors of non-communicable diseases. The country's first registry was the Shanghai Tumour Registry, which gathered information from under the age of 15 years children between 1973 and 1977.
In Japan, pharma companies have an opportunity to obtain value from real-world evidence using advanced analytics and to better ensure patient outcomes. Using advanced analytics to derive insights from RWE brings the potential benefits to a whole new level. It is expected that the globally top 20 pharma enterprises could earn an average of over USD 300 million every year for the upcoming 3–5 years by adopting improved RWE analytics in the whole value chain. This can enable pharma enterprises not exclusively to save costs but also to understand what patient qualities and behavior affect health outcomes and also to make sure that patients take the correct treatment at the right time.
Moreover, most companies in the country have already seen outcomes. A few of them are deploying advanced RWE analytics at scale, and some of them are at to begin. Advanced analytical methods in the country like machine learning, predictive models, unsupervised algorithms, and probabilistic causal models are making RWE a more effective resource for pharma companies. The RWE solutions are providing a better knowledge of patient outcomes. Thus, enhanced RWE analytics are currently gaining momentum in the country.
Furthermore, in April 2018, Japan authorized the use of the MID-NET data set, which is created by the Ministry of Health, Labor & Welfare, and the Drugs and clinical Devices Agency. This enhances electronic medical records, integrates medical claims, and analyzes gathered information from different websites. In addition, Life Data Initiative, Pfizer Japan, and NTT Data are performing joint research on the adoption of RWE to find cancer results.
Additionally, in May 2021, companies like Aetion Inc., IQVIA Inc, Flatiron Health, Tempus, and Syapse Inc. teamed up, to increase the usage of data gathered from EHRs, claims, and other sources beyond clinical trials. The alliance works closely with pharma companies, medical device manufacturers, patient groups, and other major stakeholders to promote broader initiatives to utilize RWE. Thus, such innovations will support healthcare providers to quickly access new treatment options, which, in turn, will drive market growth.
Report Breakdown
Based on Component
Based on Application
Based on End User
Geographical Analysis
The real world evidence solutions market size stood at USD 1,789.9 million in 2024.
During 2024–2030, the growth rate of the real world evidence solutions market will be around 14.2%.
Pharmaceutical & Medical Device Companies is the largest end user in the real world evidence solutions market.
The major drivers of the real world evidence solutions market include the shifting focus to value-based healthcare from volume-based, the increasing development of clinical trials, and the surging R&D activities related to the development of drugs and medical devices.
Want a report tailored exactly to your business need?
Request CustomizationLeading companies across industries trust us to deliver data-driven insights and innovative solutions for their most critical decisions. From data-driven strategies to actionable insights, we empower the decision-makers who shape industries and define the future. From Fortune 500 companies to innovative startups, we are proud to partner with organisations that drive progress in their industries.


Working with P&S Intelligence and their team was an absolute pleasure – their awareness of timelines and commitment to value greatly contributed to our project's success. Eagerly anticipating future collaborations.
McKinsey & Company
IndiaOur insights into the minutest levels of the markets, including the latest trends and competitive landscape, give you all the answers you need to take your business to new heights
We take a cautious approach to protecting your personal and confidential information. Trust is the strongest bond that connects us and our clients, and trust we build by complying with all international and domestic data protection and privacy laws
Customize the Report to Align with Your Business Objectives
Request the Free Sample Pages