Market Statistics
| Study Period | 2019 - 2030 |
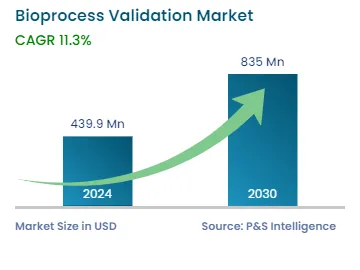
| 2024 Market Size | USD 439.9 Million |
| 2030 Forecast | USD 835 Million |
| Growth Rate(CAGR) | 11.3% |
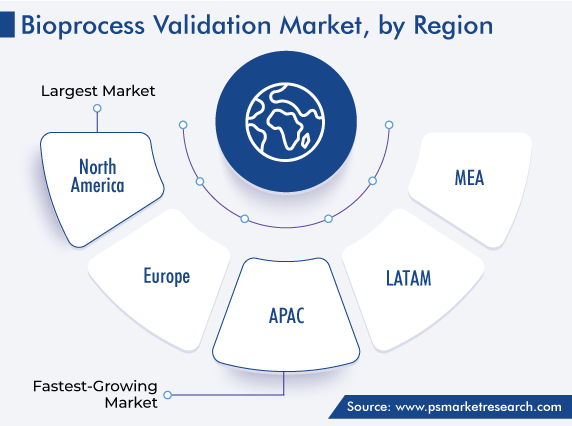
| Largest Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Nature of the Market | Fragmented |
Report Code: 12616
Get a Comprehensive Overview of the Bioprocess Validation Market Report Prepared by P&S Intelligence, Segmented by Offering (Product Type, Service Type), End User (Pharmaceutical Companies & Biotech Companies, Contract Development & Manufacturing Organizations), and Geographic Regions. This Report Provides Insights From 2019 to 2030.
| Study Period | 2019 - 2030 |
| 2024 Market Size | USD 439.9 Million |
| 2030 Forecast | USD 835 Million |
| Growth Rate(CAGR) | 11.3% |
| Largest Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Nature of the Market | Fragmented |
Explore the market potential with our data-driven report
The bioprocess validation market size stood at USD 439.9 million in 2024, and it is expected to advance at a compound annual growth rate of 11.3% during 2024–2030, to reach USD 835 million by 2030.
The growth can be primarily ascribed to the increasing outsourcing of these services and the rising stringency of the safety regulations for the healthcare industry, in order to maintain compliance with good manufacturing practices. Moreover, the growing R&D expenditure in life sciences and the increasing need to minimize the production cost across healthcare companies are expected to boost the service demand.
In addition, the use of sophisticated facilities in the pharmaceutical industry is growing at a significant rate, thereby propelling the development of the market, as are patent expirations. Essentially, the discovery and approval of novel drugs are driving the demand for the validation of bioprocesses.
Moreover, the increasing count of government policies and initiatives for the modernization of the drug regulatory pathway and speeding up the product approval procedure, the improving reimbursement policies, and the standardization of clinical studies are offering lucrative growth opportunities.
Rising Requirement for Outsourcing and Stringent Safety and Quality Standards
Quality needs to be maintained throughout the process of manufacturing bioproducts. In turn, the maintenance of quality standards involves the elimination of contaminants and impurities from chromatographic media, which contains viruses, endotoxins, nucleic acids, cell membranes, proteins, culture media components, ligands, process chemicals, product alterations aggregates, and inactive forms of microbes.
The manufacturing processes are needed to be verified with the use of a scientifically rigorous and well-documented exercise that indicates that the equipment and methods used are effective. The FDA’s General Principles of Process Validation guidelines require the building of documented evidence at all stages of bioprocess validation studies, so as to provide a high degree of assurance that the product created by this process will assure consumers of the intended product quality.
Validation is an integrated process that must follow the FDA and EMA regulations, along with other national and worldwide standards. It is aimed at the verification of all the procedures and ensure they follow all the cGMP requirements. National and international guidelines make exhaustive documentation necessary in order to confirm that the process is conducted according to the standard procedures.
In order to increase production yields, several drug makers are outsourcing to third-party service providers. The production cost can be reduced further with the use of disposable technologies during medication development. The adaptability of processes has increased with the use of single-use bioreactors, which has also significantly decreased the possibility of cross-contamination. This will benefit the market as the process reduces the commercialization duration for the products and ensures they are reliable.
Extractables/leachables testing dominates the service segment, this is attributed to the increasing risk of product adulteration and the presence of regulatory guidelines related to testing services. Among the most-prominent guidelines in this regard are those of the FDA and the current good manufacturing practice guidelines.
Individuals are also becoming increasingly concerned for the purity, safety, and quality of pharma products; therefore, they are now adopting only quality-certified bioproducts. The identification of the hazards posed by the leachables contained in packaging and closed processing systems has become essential in the drug discovery procedure, as a significant enough presence of leachables can lead to the premature perishing of bioproducts.
The bioprocess residuals testing category will grow significantly in the coming years. Bioprocess residuals include downstream impurities, cell-culture-derived upstream impurities, buffer content, and anti-foaming agents. The demand for safe, impurity-free, and quality-approved drugs, vaccines, and other therapeutic products manufactured by the biotechnology, biopharmaceutical, and pharmaceutical industries is increasing, thus propelling the category’s advance.
Filter elements are expected to grow at the highest CAGR, majorly owing to the rising usage of a variety of filters in different bioprocessing techniques. They are required to ensure the efficacy and safety of biopharmaceutical products at every step of their production.
The usage of bioreactors for the manufacturing of vaccines, medicines, and other pharmaceutical components is also burgeoning. For instance, monoclonal antibodies for the treatment of rheumatoid arthritis, cancer, and many other diseases are manufactured with the help of bioreactors. In many areas of the healthcare industry, especially research, bioreactors are used for the conversion of in-vitro 2D culture models and suspensions to the 3D form and imitating the natural physiological stateat the site.
They are also utilized to stop natural biochemical processes, by holding organisms. In a batch bioreactor, everything is added simultaneously, and the pressure and temperature are adjusted once in a controlled and sealed environment. Moreover, before opening the other system, biochemical reactions are allowed. After that, the contents can be utilized, extracted, disposed of, or further processed. It produces quality-oriented products because the conditions are accurately maintained and the product is extracted only at the end of the process.
Currently, the usage of these systems is growing for the production of viral vaccines. For instance, in May 2022, Pall Corporation announced the development of a nano bioreactor, which aids in the production of viral vectors for CAR-T therapy.
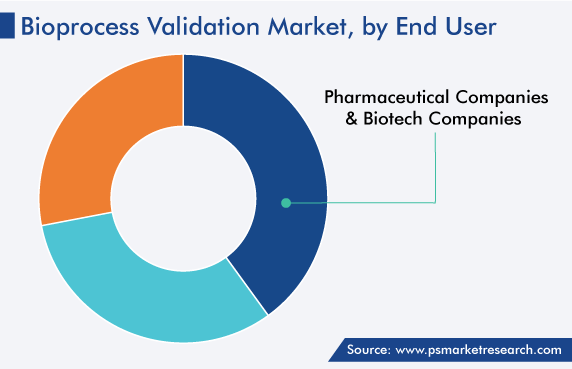
Pharmaceutical and biotechnology companies are the largest end users, ascribed to the rising requirement for biopharma products. This means that the need to check for impurities will increase, driven by the stringent regulatory standards implemented to ensure the quality and validity of the bioprocesses involved in biopharmaceutical production. The high cost of the materials used by biopharma companies for the development of various products for the treatment of individuals further encourages the development of the category.
Drive strategic growth with comprehensive market analysis
North America has the leading position in the bioprocess validation market, and it will hold the same position till 2030, with a value of USD 334 million. This is attributed to the fast adoption of new and advanced technologies and the growing production of biologics.
In North America, the U.S. holds the leading position, and it will grow with a CAGR of around 10%, attributed to the large number of key players and the rising government funding to make bioprocess validation services available to end users.
Moreover, the presence of a huge number of outsourcing service providers in the region fuels the demand. Further, the region has the existence of a large number of FDA-approved biopharmaceutical and biotechnology companies. Additionally, various novel bioprocess validation programs have been launched in the region to ensure the procedures comply with current good manufacturing practices.
In addition, the Canada and U.S. are spending increasingly on pharma/biopharma research and development. According to government websites, in 2022, the National Institutes of Health invested most of their budget of around USD 45 billion in medical research. This massive investment is forecast to boost the growth of the market in the country, itself driven by the burgeoning requirement for new active pharmaceutical ingredients and drugs.
This fully customizable report gives a detailed analysis of the bioprocess validation industry from 2019 to 2030, based on all the relevant segments and geographies.
Based on Offering
Based on End User
Geographical Analysis
The market for bioprocess validation solutions valued USD 439.9 million in 2024.
The bioprocess validation industry is driven by the rising drug quality and safety concerns and the increasing popularity of biologics.
The market for bioprocess validation solutions is dominated by pharmaceutical and biotechnology companies.
The impact of COVID-19 on the bioprocess validation industry has been positive.
Asia-Pacific will witness the highest CAGR in the market for bioprocess validation solutions.
Want a report tailored exactly to your business need?
Request CustomizationLeading companies across industries trust us to deliver data-driven insights and innovative solutions for their most critical decisions. From data-driven strategies to actionable insights, we empower the decision-makers who shape industries and define the future. From Fortune 500 companies to innovative startups, we are proud to partner with organisations that drive progress in their industries.


Working with P&S Intelligence and their team was an absolute pleasure – their awareness of timelines and commitment to value greatly contributed to our project's success. Eagerly anticipating future collaborations.
McKinsey & Company
IndiaOur insights into the minutest levels of the markets, including the latest trends and competitive landscape, give you all the answers you need to take your business to new heights
We take a cautious approach to protecting your personal and confidential information. Trust is the strongest bond that connects us and our clients, and trust we build by complying with all international and domestic data protection and privacy laws
Customize the Report to Align with Your Business Objectives
Request the Free Sample Pages