Report Code: 10934 | Available Format: PDF | Pages: 116
- Home
- Life Sciences
- Hemophilia Therapeutics Pipeline Analysis
Hemophilia Therapeutics Pipeline Analysis, 2017 - Clinical Trials & Results, Patent, Designation, Collaboration, and Other Developments
- Report Code: 10934
- Available Format: PDF
- Pages: 116
Note: This report can also be updated as of date and delivered within 1-2 working days of purchase confirmation
- Report Description
- Table of Contents
- Report Scope
- Request Free Sample
Disease Overview
Hemophilia is a genetic disorder characterized by excessive bleeding and delayed blood clotting. There are two types of hemophilia, hemophilia A (also known as classic hemophilia) and hemophilia B. According to the Centers for Disease Control and Prevention, the incidence of hemophilia stands at, one out of every 5,000 male births and as many as 20,000 males in the U.S., living with either of the two types of hemophilia, presently. The prevalence of hemophilia A is around four times high as compared to the prevalence of hemophilia B. It has been observed that the male population is more commonly affected by the disease, as compared to the female population, since hemophilia is inherited in an X-linked recessive genetic pattern, where females are generally carriers of the disease. Hemophilia A results from a deficiency of clotting Factor VIII, whereas hemophilia B (also known as the Christmas disease) is caused by a deficiency of clotting Factor IX, both of these are essential for the proper functioning of blood clotting. The cause of the deficiency of clotting factors is thought to be a mutation in one of the genes that codes for the synthesis of these clotting factor, which are required to form a blood clot.
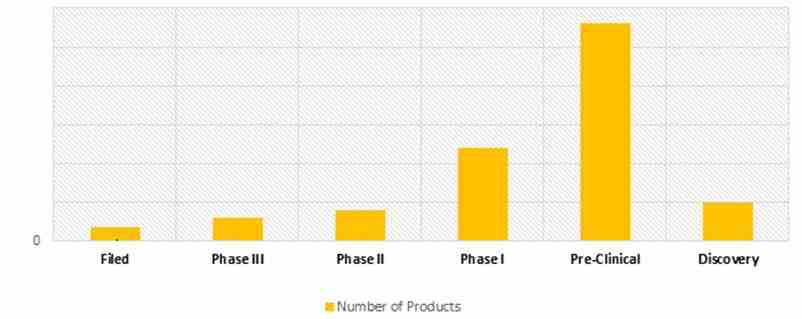
NUMBER OF HEMOPHILIA DRUG CANDIDATES UNDER DEVELOPMENT (2017)
Pipeline Analysis
Significant growth is expected in the therapeutic pipeline of hemophilia, mainly attributed to increasing collaboration between pharma as well as biotech companies, educational institutes and associations for the development of hemophilia therapeutics. Increasing prevalence of hemophilia and technological advancement are some of the major factors for the development of novel therapies for treatment of hemophilia. Positive clinical results of Phase III and Phase II drug candidates with lesser side effects are supporting the development of hemophilia therapeutics. Many awareness programs organized by pharma as well as biotechnology companies are supporting the development of hemophilia therapeutics pipeline. PolyXen is an enabling platform technology for protein drug delivery. Zinc finger nuclease mediated in vivo genome editing approach makes use of the endogenous albumin gene locus, a highly expressing and liver-specific site that can be edited with ZFNs to accept and express therapeutic genes. As of June 2017, the hemophilia pipeline comprises of approximately 53 drug candidates in the pipeline for the treatment of hemophilia in different stages of development.
Competitive Landscape
Some of the other key players developing drugs for the treatment of hemophilia include, Sangamo BioSciences, Inc., Caisson Biotech, Inc., XL-protein GmbH and others.
Scope for Customization
P&S Intelligence offers customization as per specific business requirements of clients. Illustrative customization within the scope of this report includes:
- Market Forecast – Market analysis and forecast for the drug candidates in the latest stage of development
- Company Profiles – Wider company coverage in terms of detailed analysis or additional company profiles
Get a bespoke market intelligence solution
- Buy report sections that meet your requirements
- Get the report customized as per your needs

Want a report tailored exactly to your business strategy?
Request CustomizationWant an insight-rich discussion with the report author?
Speak to AnalystOur dedication to providing the most-accurate market information has earned us verification by Dun & Bradstreet (D&B). We strive for quality checking of the highest level to enable data-driven decision making for you
Our insights into the minutest levels of the markets, including the latest trends and competitive landscape, give you all the answers you need to take your business to new heights
With 24/7 research support, we ensure that the wheels of your business never stop turning. Don’t let time stand in your way. Get all your queries answered with a simple phone call or email, as and when required
We take a cautious approach to protecting your personal and confidential information. Trust is the strongest bond that connects us and our clients, and trust we build by complying with all international and domestic data protection and privacy laws